- Joined
- Aug 15, 2000
- Messages
- 19,109
I posted this in the LGD forum in a discussion about boron slightly blueish LGD's
Diamonds with boron that are blue also conduct electricity.
That means there are free floating electrons.
Those electrons are absorbing energy in the yellow part of the spectrum.
Colorless diamonds absorb little or no energy.
Does that mean we should expect less brilliance in blue or blueish diamonds?
By comparison blue fluorescent diamonds have enhanced or additive light energy.
They collect invisible UV energy and add give off some additional blue emission.
I think this may be why I am so attracted to non hazy strong blue fluorescent diamonds in high colors - because they are actually emitting more light in the visible range.
And they have the added benefit of being a cool blue white.
Diamonds with boron that are blue also conduct electricity.
That means there are free floating electrons.
Those electrons are absorbing energy in the yellow part of the spectrum.
Colorless diamonds absorb little or no energy.
Does that mean we should expect less brilliance in blue or blueish diamonds?
By comparison blue fluorescent diamonds have enhanced or additive light energy.
They collect invisible UV energy and add give off some additional blue emission.
I think this may be why I am so attracted to non hazy strong blue fluorescent diamonds in high colors - because they are actually emitting more light in the visible range.
And they have the added benefit of being a cool blue white.




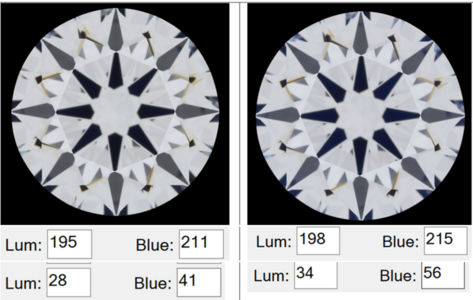


300x240.png)