- Joined
- Jun 8, 2008
- Messages
- 56,765
"

When it comes to developing long COVID, certain people may have a higher risk than others, researchers reported.
According to an online survey of people who tested positive in 2020, 52.1% said they experienced post-COVID syndrome symptoms, said Vassilios Vassiliou, PhD, of the University of East Anglia in Norwich, England, and colleagues.
One of the top predictors of exactly who might develop these long COVID symptoms was body mass index (BMI), the team reported in PLOS Global Public Health. More specifically, every 1 kg/m2 more of BMI was tied with a 3% higher relative risk for the condition (RR 1.031, 95% CI 1.016-1.047).
Female sex also appeared to be another risk factor. Compared with females, male sex was linked with a significantly lower relative risk for developing post-COVID symptoms (RR 0.748, 95% CI 0.605-0.924).
"This is really important because information like this can be used to profile those people who are 'at risk' of developing long COVID," Vassiliou explained in a statement.
The researchers also discovered that of the 25.4% of patients who utilized healthcare services after their initial COVID infection, 73.2% were experiencing symptoms of long COVID. This translated to more than a three-fold higher relative risk for people with long COVID symptoms using healthcare services (RR 3.280, 95% CI 2.540-4.262).
Similarly, males were significantly less likely to be utilizing these health services after testing positive (RR 0.618, 95% CI 0.464-0.81 . On the other hand, the same positive association with BMI was still seen (RR 1.027, 95% CI 1.009-1.046).
. On the other hand, the same positive association with BMI was still seen (RR 1.027, 95% CI 1.009-1.046).
The researchers said there was a slight trend for age per year as an independent risk factor for post-COVID symptoms, but it was not statistically significant (RR 1.003, 95% CI 0.998-1.009 per 1 year older).
"Based on the above, it can be estimated that for a man to have the same probability as a female of the same age with normal BMI, they have to have a BMI over 35 kg/m2," the researchers said.
Vassiliou added that his group hopes the study can aid policymakers in planning local COVID services, as well as inform the general public of the magnitude of the long COVID pandemic.
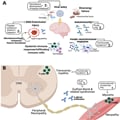
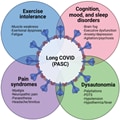
"Long COVID is a complex condition that develops during or after having COVID, and it is classified as such when symptoms continue for more than 12 weeks," he explained. "Just over two million people in the U.K. are thought to suffer with long COVID, and it affects people in different ways. Breathlessness, cough, heart palpitations, headaches, and severe fatigue are among the most prevalent symptoms.
"Other symptoms may include chest pain or tightness, brain fog, insomnia, dizziness, joint pain, depression and anxiety, tinnitus, loss of appetite, headaches, and changes to sense of smell or taste," Vassiliou added.
A total of 1,487 individuals residing in East England completed the online survey. Post-COVID syndrome was defined as individuals who selected one or more new self-reported symptoms from the questionnaire following COVID illness. A total of 61% of the cohort were females, average age was 50, and average BMI was 28.4.
Because the study included only individuals with a confirmed COVID-19 infection between the start of the pandemic through December 6, 2020, the vast majority of participants were unvaccinated at the time of infection, the researchers noted. Only 11 participants had received their first vaccine dose.
"All of these people were infected in the months before the COVID vaccination program was rolled out and they suffered from numerous new symptoms that were not present before their COVID infection," said Vassiliou.
Vassiliou and co-authors reported no disclosures.
Primary Source
PLOS Global Public Health
Source Reference: Debski M, et al "Post-COVID-19 syndrome risk factors and further use of health services in East England" PLOS Glob Public Health 2022; DOI: 10.1371/journal.pgph.0001188.
"
Higher BMI a Risk Factor for Long COVID Symptoms
— Female sex was another independent risk factor, U.K. study showed
by Kristen Monaco, Staff Writer, MedPage Today November 30, 2022
When it comes to developing long COVID, certain people may have a higher risk than others, researchers reported.
According to an online survey of people who tested positive in 2020, 52.1% said they experienced post-COVID syndrome symptoms, said Vassilios Vassiliou, PhD, of the University of East Anglia in Norwich, England, and colleagues.
One of the top predictors of exactly who might develop these long COVID symptoms was body mass index (BMI), the team reported in PLOS Global Public Health. More specifically, every 1 kg/m2 more of BMI was tied with a 3% higher relative risk for the condition (RR 1.031, 95% CI 1.016-1.047).
Female sex also appeared to be another risk factor. Compared with females, male sex was linked with a significantly lower relative risk for developing post-COVID symptoms (RR 0.748, 95% CI 0.605-0.924).
"This is really important because information like this can be used to profile those people who are 'at risk' of developing long COVID," Vassiliou explained in a statement.
The researchers also discovered that of the 25.4% of patients who utilized healthcare services after their initial COVID infection, 73.2% were experiencing symptoms of long COVID. This translated to more than a three-fold higher relative risk for people with long COVID symptoms using healthcare services (RR 3.280, 95% CI 2.540-4.262).
Similarly, males were significantly less likely to be utilizing these health services after testing positive (RR 0.618, 95% CI 0.464-0.81
The researchers said there was a slight trend for age per year as an independent risk factor for post-COVID symptoms, but it was not statistically significant (RR 1.003, 95% CI 0.998-1.009 per 1 year older).
"Based on the above, it can be estimated that for a man to have the same probability as a female of the same age with normal BMI, they have to have a BMI over 35 kg/m2," the researchers said.
Vassiliou added that his group hopes the study can aid policymakers in planning local COVID services, as well as inform the general public of the magnitude of the long COVID pandemic.
"Long COVID is a complex condition that develops during or after having COVID, and it is classified as such when symptoms continue for more than 12 weeks," he explained. "Just over two million people in the U.K. are thought to suffer with long COVID, and it affects people in different ways. Breathlessness, cough, heart palpitations, headaches, and severe fatigue are among the most prevalent symptoms.
"Other symptoms may include chest pain or tightness, brain fog, insomnia, dizziness, joint pain, depression and anxiety, tinnitus, loss of appetite, headaches, and changes to sense of smell or taste," Vassiliou added.
A total of 1,487 individuals residing in East England completed the online survey. Post-COVID syndrome was defined as individuals who selected one or more new self-reported symptoms from the questionnaire following COVID illness. A total of 61% of the cohort were females, average age was 50, and average BMI was 28.4.
Because the study included only individuals with a confirmed COVID-19 infection between the start of the pandemic through December 6, 2020, the vast majority of participants were unvaccinated at the time of infection, the researchers noted. Only 11 participants had received their first vaccine dose.
"All of these people were infected in the months before the COVID vaccination program was rolled out and they suffered from numerous new symptoms that were not present before their COVID infection," said Vassiliou.
-
![author['full_name'] author['full_name']](https://clf1.medpagetoday.com/media/images/author/kristenMonaco_188.jpg)
Kristen Monaco is a staff writer, focusing on endocrinology, psychiatry, and nephrology news. Based out of the New York City office, she’s worked at the company since 2015.
Vassiliou and co-authors reported no disclosures.
Primary Source
PLOS Global Public Health
Source Reference: Debski M, et al "Post-COVID-19 syndrome risk factors and further use of health services in East England" PLOS Glob Public Health 2022; DOI: 10.1371/journal.pgph.0001188.
"








![author['full_name'] author['full_name']](https://clf1.medpagetoday.com/media/images/author/kristinaFiore_188.jpg)
















300x240.png)