- Joined
- Jun 8, 2008
- Messages
- 54,785
Happy March everyone. And accordingly we continue "march"ing on during the pandemic. One day at a time. I hope we experience more happy updates and promising news for the hopefully soon (by end of this year maybe?) end of the pandemic.
 www.cnn.com
www.cnn.com
"
and
 www.cnn.com
www.cnn.com

5 things to know for March 1: Covid-19, stimulus, immigration, Middle East, Myanmar | CNN
Here's what else you need to know to Get Up to Speed and On with Your Day.
"
1. Coronavirus
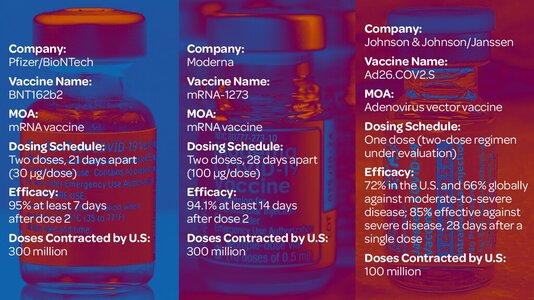
The US Food and Drug Administration has given emergency use authorization to the Johnson & Johnson coronavirus vaccine, and the Centers for Disease Control and Prevention chief signed off on its advisers' nod of approval. Now, 3.9 million doses of the third US Covid-19 vaccine are making their way across the country. The J&J vaccine is different than the other two because it only requires one dose. Which should you get? Dr. Anthony Fauci says, "I would take whatever vaccine would be available to me as quickly as possible." Today, Mexico's President is expected to ask President Biden about the US possibly sharing its vaccine supply. Mexico has several purchase agreements with drug makers, but many have gone unfulfilled. Meanwhile, more than 2,400 cases of the UK, Brazil and South Africa coronavirus variants have been detected in the US, and the CDC warns the actual number could be much higher."and

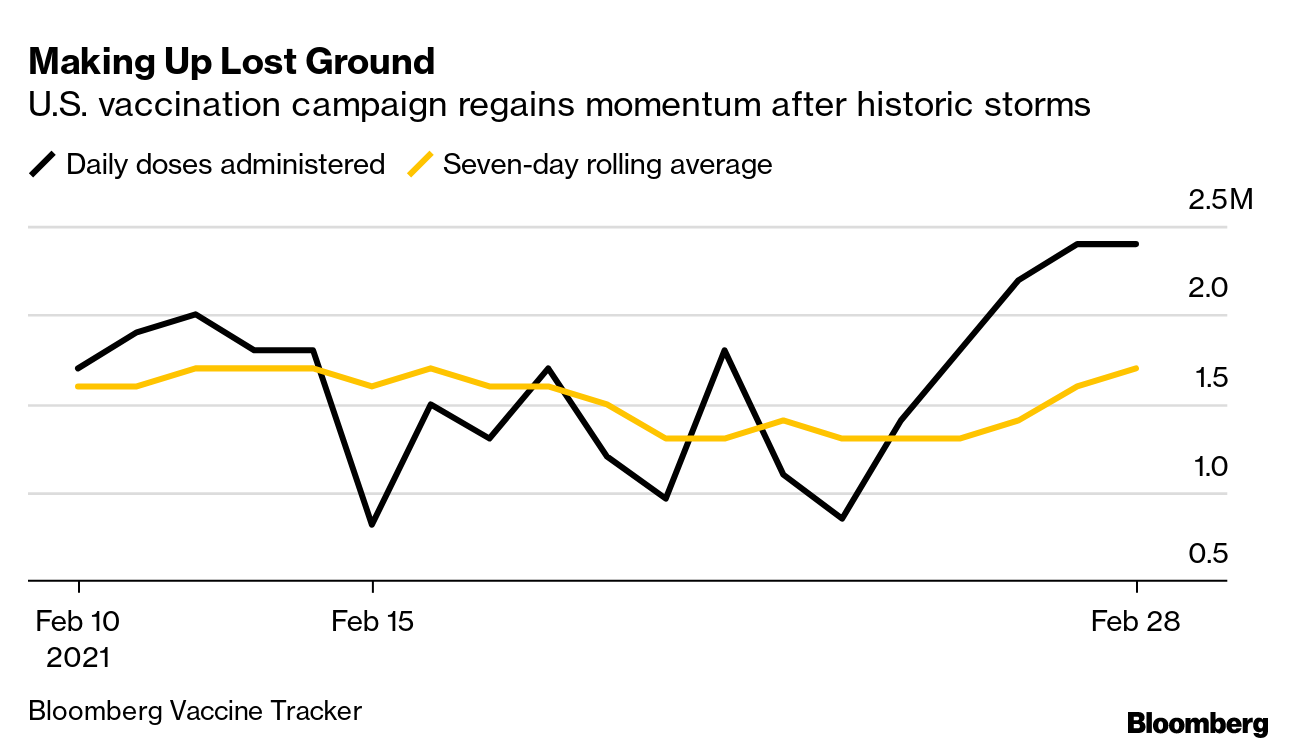
March 1, 2021 coronavirus news
The coronavirus pandemic has brought countries to a standstill. Meanwhile, vaccinations have already started in some countries as cases continue to rise. Follow here for the latest.













300x240.png)