zeolite
Brilliant_Rock
- Joined
- Aug 13, 2008
- Messages
- 619
https://www.pricescope.com/community/threads/would-you-recut-this.110168/
I''m referring to this thread, but wanted to start a new one, since this diverges from the topic of cutting the amethyst.
Any gemstone will appear to change color slightly when viewed in different light. Color change and color shift differ only in the amount of change. Color change is strong, while color shift is weaker.
Both of these terms refer to changes in color of the same gem, viewed in the same direction under different light. Different light often refers to sunlight which is stronger in blue wavelengths, while artificial light, usually incandescent bulbs, are strong in orange and red light.
The dividing line between a normal gem, a color shift gem and a color change gem is not clearly defined. But buyers, who are able to view many gems, especially fine alexandrites, in different types of lighting, will have some idea of what is a color shift gem and what is a color change gem.
FrekeChild: Color change amethyst? Never heard of it.
Cofor is right, this is a marketing trick, exploiting purple and different light sources. All purple gems will be at least a color shift gem (some can be color change). Let me explain the physics:
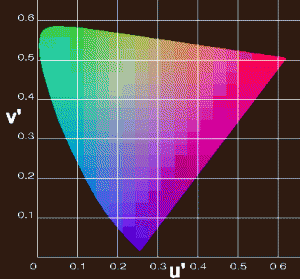
The color diagram below is the CIE Chromaticity diagram. It defines color in a very exact and repeatable way. Physicists use a color instrument, called a spectrophotometer, which can measure the wavelength of light in extremely tiny (nanometer) wavelength increments. If the color is very pastel, it will be plotted closer to the center of this diagram. If the color is very pure and saturated, it will be plotted inside the diagram, but very close to the outline of the two curved sides of this diagram. The intensity of fine ruby, sapphire, and emerald is very high and their color will be plotted very close to the outline of the diagram. The one straight side is a forbidden zone, which I''ll explain below.
Please bear with me on my names of colors; it is required for the physics below. At the bottom of the diagram, the shortest wavelength our eye can see is violet. As we travel to the upper left, we plot blue, then at the upper left curved point, green, then yellow, orange, and red. The importance of this curve is that each color can be defined by one single wavelength.
Guess what color is not on the curve? Purple. In the physical sense, purple is not a color. That is, it can''t be represented by one wavelength. All of the colors around the two curved sides of the triangle can be created by one wavelength.
So where is purple on this diagram? It is in the middle of the one straight side of the diagram (the forbidden zone), because purple is made up of both red and blue wavelengths.
So how does this relate to purple gems and color change? Daylight is relatively strong in blue wavelengths. Incandescent light is strong on red. Purple gems viewed in daylight will appear to the blue side of purple (violet). When viewed in incandescent lighting, the same gem will appear to the red side of purple (magenta).
So all purple gems (including amethyst, sapphire and especially tanzanite) will appear to have some amount of color shift. Yes, color change amethyst is a marketing trick.
I''m referring to this thread, but wanted to start a new one, since this diverges from the topic of cutting the amethyst.
Any gemstone will appear to change color slightly when viewed in different light. Color change and color shift differ only in the amount of change. Color change is strong, while color shift is weaker.
Both of these terms refer to changes in color of the same gem, viewed in the same direction under different light. Different light often refers to sunlight which is stronger in blue wavelengths, while artificial light, usually incandescent bulbs, are strong in orange and red light.
The dividing line between a normal gem, a color shift gem and a color change gem is not clearly defined. But buyers, who are able to view many gems, especially fine alexandrites, in different types of lighting, will have some idea of what is a color shift gem and what is a color change gem.
FrekeChild: Color change amethyst? Never heard of it.
Cofor: I think the color change is a sales trick. E-bay sellers keep on inventing their own nomenclature to make things more attractive. The "change" is just the ordinary difference in hue of deep colored amethyst, depending on lightning type. Bluish violet in daylight and more reddish in incandescent light.
Cofor is right, this is a marketing trick, exploiting purple and different light sources. All purple gems will be at least a color shift gem (some can be color change). Let me explain the physics:
The color diagram below is the CIE Chromaticity diagram. It defines color in a very exact and repeatable way. Physicists use a color instrument, called a spectrophotometer, which can measure the wavelength of light in extremely tiny (nanometer) wavelength increments. If the color is very pastel, it will be plotted closer to the center of this diagram. If the color is very pure and saturated, it will be plotted inside the diagram, but very close to the outline of the two curved sides of this diagram. The intensity of fine ruby, sapphire, and emerald is very high and their color will be plotted very close to the outline of the diagram. The one straight side is a forbidden zone, which I''ll explain below.
Please bear with me on my names of colors; it is required for the physics below. At the bottom of the diagram, the shortest wavelength our eye can see is violet. As we travel to the upper left, we plot blue, then at the upper left curved point, green, then yellow, orange, and red. The importance of this curve is that each color can be defined by one single wavelength.
Guess what color is not on the curve? Purple. In the physical sense, purple is not a color. That is, it can''t be represented by one wavelength. All of the colors around the two curved sides of the triangle can be created by one wavelength.
So where is purple on this diagram? It is in the middle of the one straight side of the diagram (the forbidden zone), because purple is made up of both red and blue wavelengths.
So how does this relate to purple gems and color change? Daylight is relatively strong in blue wavelengths. Incandescent light is strong on red. Purple gems viewed in daylight will appear to the blue side of purple (violet). When viewed in incandescent lighting, the same gem will appear to the red side of purple (magenta).
So all purple gems (including amethyst, sapphire and especially tanzanite) will appear to have some amount of color shift. Yes, color change amethyst is a marketing trick.











300x240.png)