- Joined
- Mar 11, 2013
- Messages
- 2,462
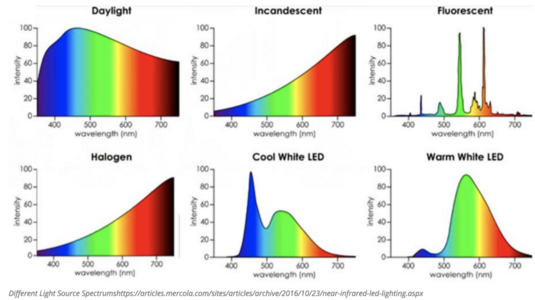
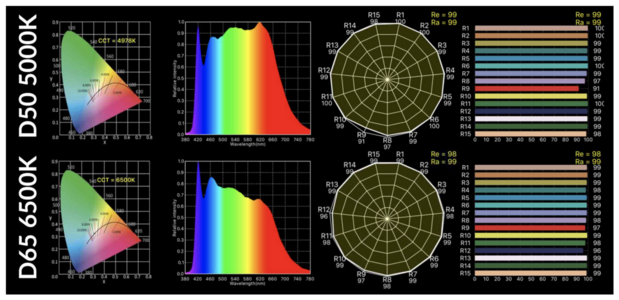
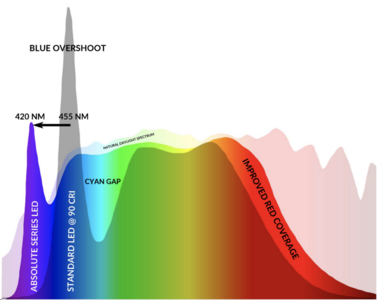
Wby do sapphires change color in different kinds of light?
I have, for example, a bezeled sapphire (sold to me as Natural Ceylon - not a definitive description as it has no cert) that is a very transparent unsaturated grayish blue in dim indoor light. In regular indoor light the stone appears more saturated and starts to take on a more deep electric blue where indirect light hits it. In direct outdoor light the stone is transparent and bright electric blue with no purple tint. In shaded outdoor light it becomes saturated loses transparency and is very deep saturated blue with a purple undertone. In the car it is deep intense saturated blue.
I’ve seen a thread here on a nearly transparent antique cut sapphire that changes color in various kinds of light; my sapphire is similar.
Is this something that natural sapphires only do, or can lab created sapphires do that as well? Do heated sapphires change color the way unheated sapphires do? Is there some sort of internal mechanism at play (like microscopic inclusions that reflect various wave lengths of light)? Thanks for any info you might have.
I have, for example, a bezeled sapphire (sold to me as Natural Ceylon - not a definitive description as it has no cert) that is a very transparent unsaturated grayish blue in dim indoor light. In regular indoor light the stone appears more saturated and starts to take on a more deep electric blue where indirect light hits it. In direct outdoor light the stone is transparent and bright electric blue with no purple tint. In shaded outdoor light it becomes saturated loses transparency and is very deep saturated blue with a purple undertone. In the car it is deep intense saturated blue.
I’ve seen a thread here on a nearly transparent antique cut sapphire that changes color in various kinds of light; my sapphire is similar.
Is this something that natural sapphires only do, or can lab created sapphires do that as well? Do heated sapphires change color the way unheated sapphires do? Is there some sort of internal mechanism at play (like microscopic inclusions that reflect various wave lengths of light)? Thanks for any info you might have.








300x240.png)