drk14
Brilliant_Rock
- Joined
- Jun 25, 2014
- Messages
- 1,061
Is that your only possible objection?
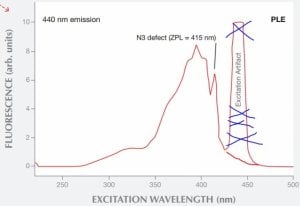
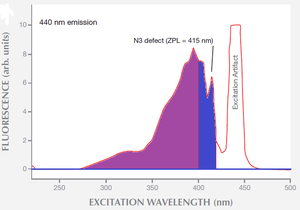
Another problem with the graph you are using is that these spectra were measured at near liquid nitrogen temperature (-196°C), so they will not be directly relevant to room-temperature observations. Cooling the diamonds to cryogenic temperatures results in significant quenching of the phonon sidebands in both the absorption spectrum and the emission spectrum. This completely alters the shape and amplitude of the spectra, compared to what would happen at room temperature.





300x240.png)