- Joined
- Jul 27, 2009
- Messages
- 4,096
Re: Article: Over Grading of Blue Fluorescent Diamonds Revis
By the way, where did you ever get the idea that I am "disputing that the grade whitening phenomena exists"? You are not paying attention.
Yes, and if the tools fail to verify what you think you're seeing, then what? There are a few possibilities including that the tools are faulty or that we may not be seeing what have always thought we were seeing.Rockdiamond|1456271436|3994650 said:There's absolutely no one who ( other than you) who is disputing the grade whitening phenomena exists.
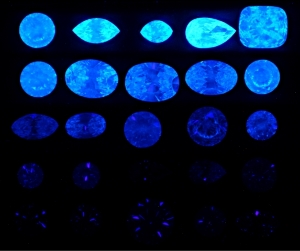
Besides everything Garry just mentioned, I promise, I did not make up the fact Harry Winston used to get a premium for diamonds that had this characteristic.
They were called "Premier"- I can't find a way to look it up, but it's a fact.
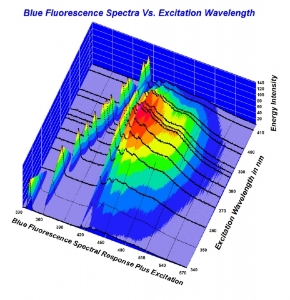
To me - a person who has great respect for science, and an inquiring mind- but not to the extent I want to take the effort necessary to understand the nifty graphs that are being displayed.... it's such a moot point as to why.
But I respect those like Garry- and Michael- who take time to research these aspects. Clearly there's a lot to learn- but it all begins with physical observation. As opposed to trying to measure light with tools. Using the tools to verify what we see makes sense.
Trying to interpret them to dispute a physical reality seems off target.
By the way, where did you ever get the idea that I am "disputing that the grade whitening phenomena exists"? You are not paying attention.

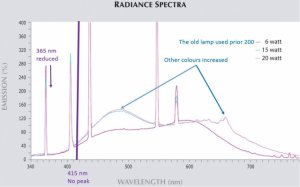
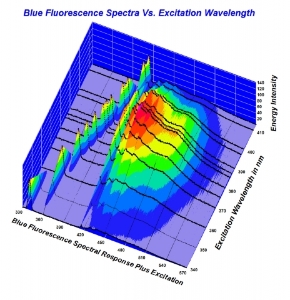
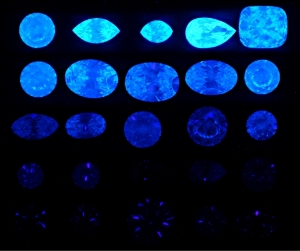
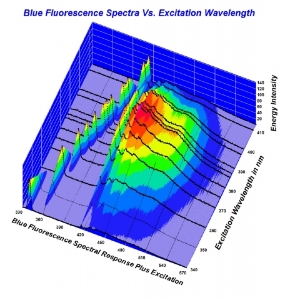
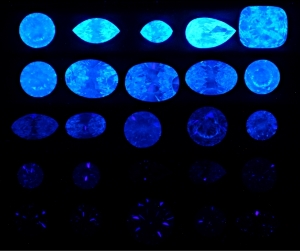
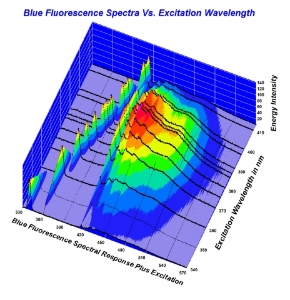
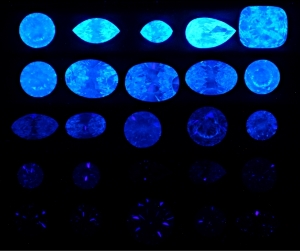
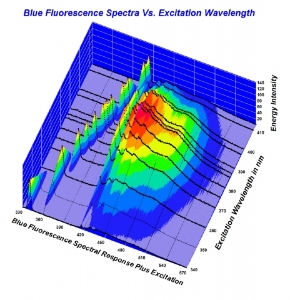



300x240.png)