- Joined
- Aug 15, 2000
- Messages
- 18,723
As a diamond dude with an interest and love of blue fluorescent diamonds, I know that cheap LED UV lights create a Strong UV effect in diamonds that GIA grades as Faint.
This also applies to many colored gems.
One of my real scientist friends who is also a gemologist wrote this email to me today when we were discussing the diamond issue.
Paraphrased from Grant Pearson FGAA:
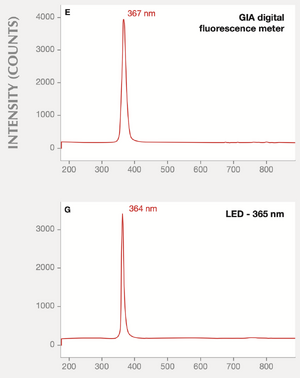
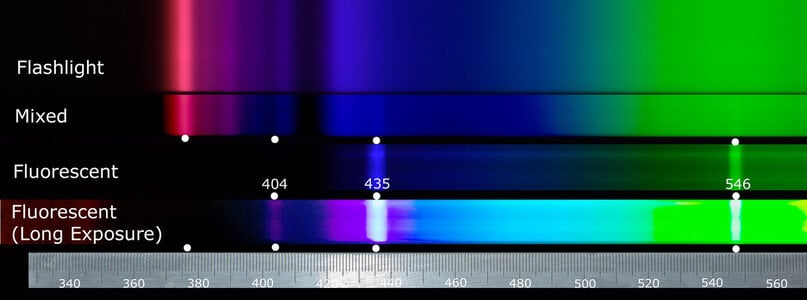
"....for instance the red chromic fluorescence of ruby, red spinel & even of alexandrite etc. The erroneous use of the supposed classic 365nm LWUV wavelength mercury vapor tube has long thought to be the effective exciting wavelength.
From my own investigations the effective actual active excitation even for exciting ruby red fluorescence from an ordinary LWUV tube is a longer-wavelength minor emission because there is some about 395nm or so in the output. The effect is not from the long-supposed 365nm which is next to useless actually."
Back to diamonds - when you use one of these more expensive (still cheap) 405nm visible violet laser pointers almost all diamonds fluoresce - so much for GIA claims that about a third fluoresce!

This also applies to many colored gems.
One of my real scientist friends who is also a gemologist wrote this email to me today when we were discussing the diamond issue.
Paraphrased from Grant Pearson FGAA:
"....for instance the red chromic fluorescence of ruby, red spinel & even of alexandrite etc. The erroneous use of the supposed classic 365nm LWUV wavelength mercury vapor tube has long thought to be the effective exciting wavelength.
From my own investigations the effective actual active excitation even for exciting ruby red fluorescence from an ordinary LWUV tube is a longer-wavelength minor emission because there is some about 395nm or so in the output. The effect is not from the long-supposed 365nm which is next to useless actually."
Back to diamonds - when you use one of these more expensive (still cheap) 405nm visible violet laser pointers almost all diamonds fluoresce - so much for GIA claims that about a third fluoresce!






300x240.png)