- Joined
- Feb 26, 2003
- Messages
- 379
Re: Article: Over Grading of Blue Fluorescent Diamonds Revis
With an advanced degree in electronic communications, you might think that I would be more skilled in communicating diamond grading and other gemological concepts to the consumer. But that form of communication is a very different skill, that of a writer and journalist.
Graduate Gemologist Richard Wise is an expert in this form of communication, and he has applied his writing skills to the field of gems and the gem trade in his newly published book "Secrets of the Gem Trade, Second Edition”.
His unique skills in journalism combined with extensive academic and real world knowledge and experience in gems and jewelry have resulted in an outstanding very readable work for the consumer public. “Secrets” reads like a novel as it communicates to the gem and jewelry lover sometimes technical and scientific information along with the secrets, romance and beauty of the diverse and wonderful world of gems. The gorgeous imagery throughout this work is icing on a delicious book of gems that encompasses essential aspects of the whole gem trade, from mine to jeweler.
I post this review here in hopes that those following this thread on blue fluorescent diamonds will take advantage of and enhance their knowledge and understanding of this subject from his perspective in the three chapters “Blue-white Diamonds”, “Colorless Diamonds”, and “Golconda or Type IIa Diamonds”.
Here are a couple of relevant excerpts that are fleshed out in these chapters.
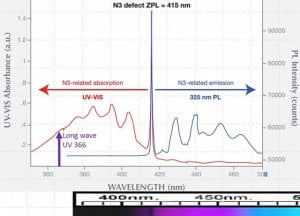
“Historically, highly transparent diamonds with white and bluish white body color were considered gems of the finest water, a rare quality known as river. In the late 19th century, fully one hundred years after the Indian mines were tapped out, diamonds from southern Africa began to flood the market. Some of these were blue fluorescent diamonds. According to the legendary gemologist, Frank Wade, writing in 1915, those that did not bleed color, that is, did not appear yellowish face up in the low ultraviolet of incandescent light were added to the river category.”
“What does this mean for the connoisseur? River quality stones are very rare, but these original blue white type IIa’s and blue fluorescent diamonds that hold their color (in low UV light) are well worth the seeking and likely to be priced well below their true value.”
Check out: www.secretsofthegemtrade.com..... The Introduction and Overview to Blue White Diamonds is one of the sample chapters on the website. ( http://secretsofthegemtrade.com/?page_id=32 )

With an advanced degree in electronic communications, you might think that I would be more skilled in communicating diamond grading and other gemological concepts to the consumer. But that form of communication is a very different skill, that of a writer and journalist.
Graduate Gemologist Richard Wise is an expert in this form of communication, and he has applied his writing skills to the field of gems and the gem trade in his newly published book "Secrets of the Gem Trade, Second Edition”.
His unique skills in journalism combined with extensive academic and real world knowledge and experience in gems and jewelry have resulted in an outstanding very readable work for the consumer public. “Secrets” reads like a novel as it communicates to the gem and jewelry lover sometimes technical and scientific information along with the secrets, romance and beauty of the diverse and wonderful world of gems. The gorgeous imagery throughout this work is icing on a delicious book of gems that encompasses essential aspects of the whole gem trade, from mine to jeweler.
I post this review here in hopes that those following this thread on blue fluorescent diamonds will take advantage of and enhance their knowledge and understanding of this subject from his perspective in the three chapters “Blue-white Diamonds”, “Colorless Diamonds”, and “Golconda or Type IIa Diamonds”.
Here are a couple of relevant excerpts that are fleshed out in these chapters.
“Historically, highly transparent diamonds with white and bluish white body color were considered gems of the finest water, a rare quality known as river. In the late 19th century, fully one hundred years after the Indian mines were tapped out, diamonds from southern Africa began to flood the market. Some of these were blue fluorescent diamonds. According to the legendary gemologist, Frank Wade, writing in 1915, those that did not bleed color, that is, did not appear yellowish face up in the low ultraviolet of incandescent light were added to the river category.”
“What does this mean for the connoisseur? River quality stones are very rare, but these original blue white type IIa’s and blue fluorescent diamonds that hold their color (in low UV light) are well worth the seeking and likely to be priced well below their true value.”
Check out: www.secretsofthegemtrade.com..... The Introduction and Overview to Blue White Diamonds is one of the sample chapters on the website. ( http://secretsofthegemtrade.com/?page_id=32 )

















300x240.png)