Daisys and Diamonds
Super_Ideal_Rock
- Joined
- Apr 30, 2019
- Messages
- 24,739
Neither would I! I eat out heaps and go shopping with no mask but a crowded train with no mask is a bridge too far for me.
I caught a train earlier in the year that was packed with people coughing. I was convinced I would catch Covid from it (seriously you could practically see the germs) but I didn't.
I do wonder what infection though. I was carrying and kissing my Covid ridden child and I never caught it.
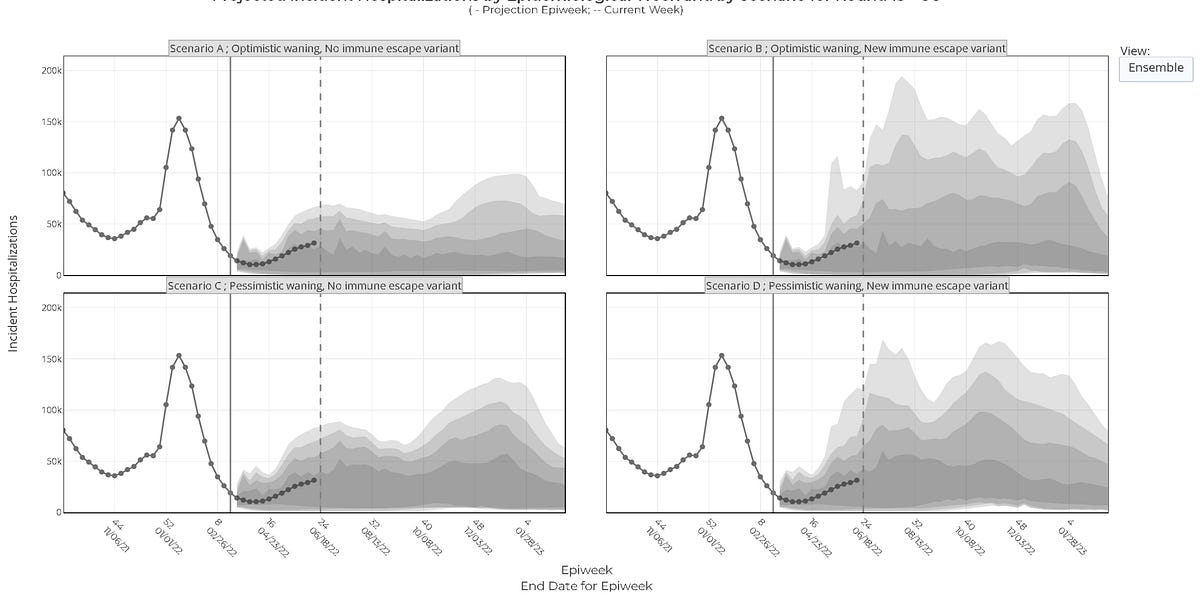
There are still lots of us who havn't caught it
Are we lucky ?
Or just careful ?
Or some other reason ?









300x240.png)